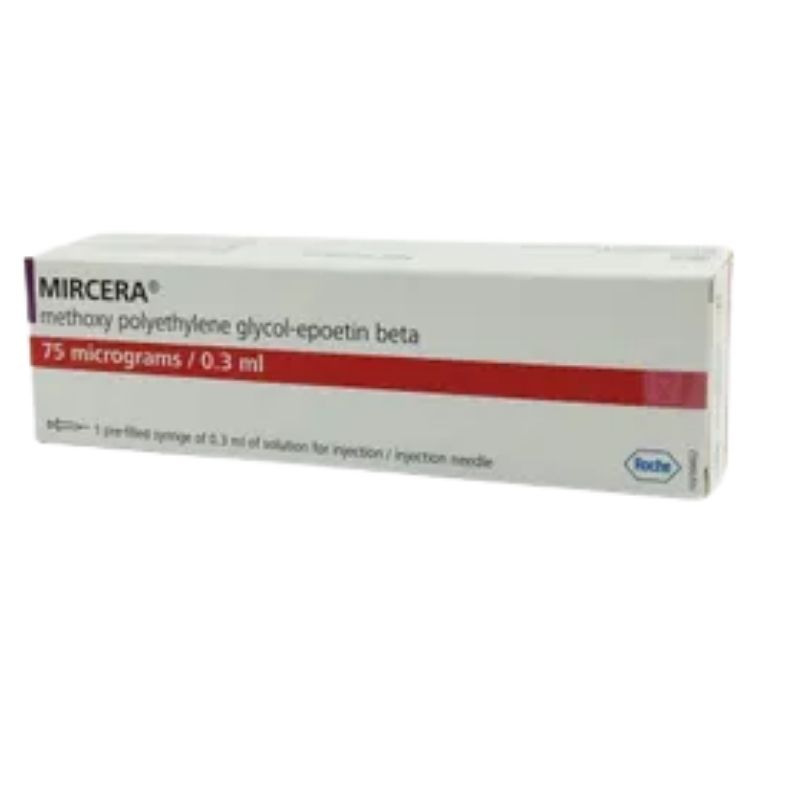
Mircera 75 Injection – (75mcg/0.3ml)
9,622.00৳ Original price was: 9,622.00৳ .9,233.00৳ Current price is: 9,233.00৳ .
Radiant Pharmaceuticals Ltd.
Generic: Methoxy Polyethyelene Glycol-Epoetin Beta
1 Injection
| Weight | 0.015 kg |
|---|
0
People watching this product now!
Description
Indication
Anaemia associated with chronic kidney disease
Administration
IV route is recommended for patients receiving hemodialysis because IV route may be less immunogenic SC: inject in abdomen, arm or thigh
Adult Dose
Patients with chronic renal failure on dialysis Initiate treatment when hemoglobin level < 10 g/dL If hemoglobin level approaches or exceeds 11 g/dL, reduce or interrupt dose Dose if not currently on ESA therapy: 0.6 mcg/kg IV/SC q2week initially Once hemoglobin has been stabilized, dose may be administered once monthly using a dose that is twice that of every-two-week dose and subsequently titrated as necessary Patients with chronic renal failure not on dialysis Consider initiating treatment only when hemoglobin level is < 10 g/dL and the following considerations apply. Rate of hemoglobin decline indicates likelihood of requiring a RBC transfusion…
Child Dose
Safety & efficacy not established
Contraindication
Hypersensitivity. Uncontrolled HTN.
Mode of Action
Methoxy Polyethyelene Glycol-Epoetin Beta is an erythropoietin receptor activator w/ greater activity as well as increased half-life, in contrast to erythropoietin.
Precaution
Discontinue if pure red cell aplasia. Adequate control of BP. Hemoglobinopathies, seizures, platelet level >500 x 109/L. Patients <18 yr. Lactation: not known if excreted in breast milk, use caution
Side Effect
>10% Hypertension (13%),Diarrhea (11%),Nasopharyngitis (11%) 1-10% Headache (9%),Upper respiratory tract infection (9%),Cough (6%),Hypotension (5%),Urinary tract infection (5%),Procedural arteriovenous fistula thrombosis (5%) Frequency Not Defined Coronary artery disease,Anemia,Septic shock,Serious cardiovascular and thromboembolic events,Seizures,Immunogenicity related PRCA,Increased mortality and/or tumor progression in cancer patients,Increased mortality,Concomitant termination of other CRF therapy,Stevens-Johnson syndrome,Toxic epidermal necrolysis
Interaction
No interaction studies have been performed. There is no evidence that Mircera alters the metabolism of other medicinal products.
Reviews (0)
Only logged in customers who have purchased this product may leave a review.




Reviews
There are no reviews yet.