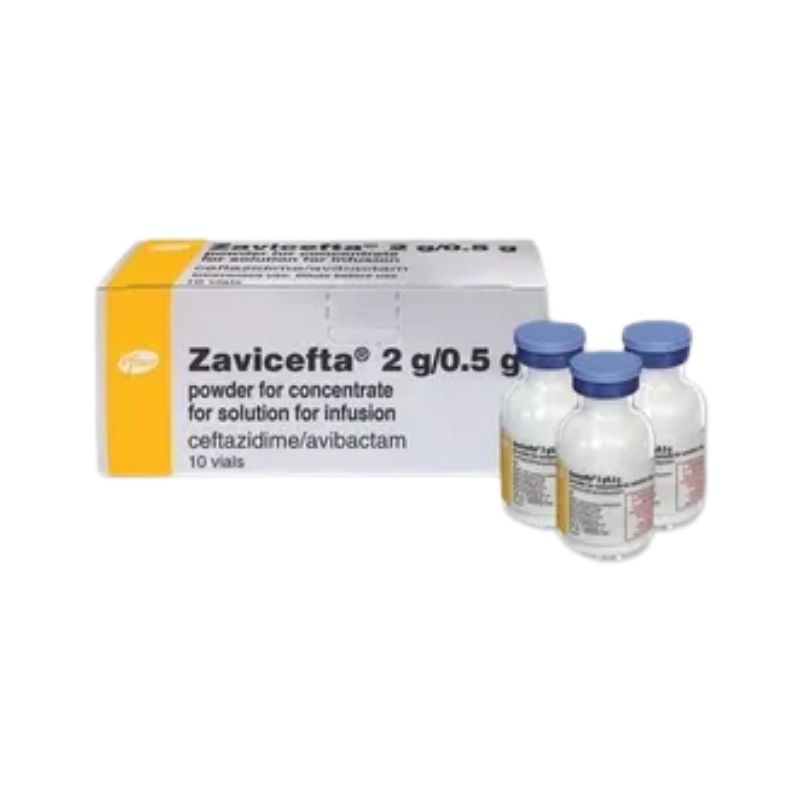
Zavicefta Injection – (2gm+0.5gm/Vial)
6,460.00৳ Original price was: 6,460.00৳ .6,199.00৳ Current price is: 6,199.00৳ .
Radiant Pharmaceuticals Ltd.
Generic: Ceftazidime + Avibactam
1 x 2.5gm Vial
| Weight | 0.1 kg |
|---|
Out of stock
0
People watching this product now!
Description
Indication
Intra-abdominal Infections, Urinary Tract Infections, Hospital-acquired and ventilator-associated bacterial pneumonia
Administration
IV Preparation Reconstitute powder with 10 mL of one of the following solutions Sterile water for injection 0.9% NaCl D5W All combinations of dextrose and sodium chloride injection containing up to 2.5% dextrose and 0.45% NaCl Lactated Ringer injection Upon constitution, solution may be held for no longer than 30 minutes before transferring and further diluting in a suitable infusion bag Additional dilution required Mix vial gently with 10 mL of a solution listed above Reconstituted solution results in ~0.167 g/mL of ceftazidime and ~0.042 g/mL avibactam Further dilute with the same diluent used for reconstitution (except sterile water for…
Adult Dose
Intra-abdominal Infections Indicated in combination with metronidazole for complicated intra-abdominal infections (cIAIs) caused by the following susceptible gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter freundii complex, and Pseudomonas aeruginosa 2.5 g (2 g/0.5 g) IV q8hr infused over 2 hr for 5-14 days Urinary Tract Infections Indicated for complicated urinary tract infections (cUTIs) including pyelonephritis caused by the following susceptible microorganisms: Escherichia coli, Klebsiella pneumoniae, Citrobacter koseri, Enterobacter aerogenes, Enterobacter cloacae, Citrobacter freundii, Proteus mirabilis, and Pseudomonas aeruginosa 2.5 g (2 g/0.5 g) IV q8hr infused over 2 hr for 7-14 days Bacteria Pneumonia…
Child Dose
<18 years: Safety and efficacy not established
Renal Dose
Renal impairment CrCl >50 mL/min: No dose adjustment required CrCl 31-50 mL/min: 1.25 g (1 g/0.25 g) IV q8hr CrCl 16-30 mL/min: 0.94 g (0.75 g/0.19 g) IV q12hr CrCl 6-15 mL/min: 0.94 g (0.75 g/0.19 g) IV q24hr CrCl <5 mL/min: 0.94 g (0.75 g/0.19 g) IV q48hr Patients on hemodialysis: Administer after hemodialysis on hemodialysis days
Contraindication
Known serious hypersensitivity to avibactam, ceftazidime, or other cephalosporins
Mode of Action
Ceftazidime: Third-generation cephalosporin with broad-spectrum gram-negative activity, including Pseudomonas; arrests bacterial growth by binding to 1 or more penicillin-binding proteins, and thereby inhibiting final transpeptidation step of peptidoglycan synthesis in bacterial cell-wall synthesis and inhibiting cell-wall biosynthesis Avibactam: Diazabicyclooctanone, non-beta-lactam, beta-lactamase inhibitor; alone has no antibacterial activity at clinically relevant doses; when combined with ceftazidime, avibactam protects ceftazidime from degradation by beta-lactamase enzymes and effectively extends the antibiotic spectrum of ceftazidime to include many gram-negative bacteria normally not susceptible to ceftazidime
Precaution
Serious and occasionally fatal anaphylactic reactions and serious skin reactions have been reported with beta-lactam antibacterials; caution in patients with history of allergy to cephalosporins, penicillins, or carbapenems Clostridium difficile associated diarrhea (CDAD) has been associated with nearly all systemic antibacterials; severity may range from mild diarrhea to fatal colitis; may occur >2 months following antibacterial use; if CDAD suspected, manage fluid and electrolytes levels and monitor antibacterial treatment for CDAD Seizures, nonconvulsive status epilepticus, encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia have been reported with ceftazidime, especially in the setting of renal impairment; adjust dose according to CrCl (see…
Side Effect
1-10% (in cIAI plus metronidazole) Diarrhea (8%) Nausea (7%) Vomiting (5%) Headache (3%) Dizziness (2%) Abdominal pain (1%) 1-10% (in cUTI) Diarrhea (3%) Nausea (3%) Constipation (2%) Upper abdominal pain (1%) 1-10% (in HABP/VABP) Nausea (3%) Pruritus (2%) Frequency Not Reported Blood and lymphatic disorders: Thrombocytopenia, thrombocytosis, leukopenia General disorders and administration site conditions: Injection site phlebitis Infections and infestations: Candidiasis Investigations:Increased aspartate aminotransferase, increased alanine aminotransferase, increased gamma-glutamyltransferase Metabolism and nutrition disorders: Hypokalemia Nervous system disorders: Dysgeusia Renal and urinary disorders: Acute kidney injury, renal impairment, nephrolithiasis Skin and subcutaneous tissue disorders: Rash, rash maculopapular, urticaria Psychiatric disorders: Anxiety
Pregnancy Category Note
Pregnancy There are no adequate and well-controlled studies of ceftazidime/avibactam, ceftazidime, or avibactam in pregnant women Neither ceftazidime nor avibactam were teratogenic in rats at doses 40 and 9 times the recommended human clinical dose; in the rabbit, at twice the exposure as seen at the human clinical dose, there were no effects on embryofetal development with avibactam Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed Lactation Ceftazidime is excreted in human milk in low concentrations Unknown whether avibactam is excreted into human milk, although avibactam…
Reviews (0)
Only logged in customers who have purchased this product may leave a review.



Reviews
There are no reviews yet.