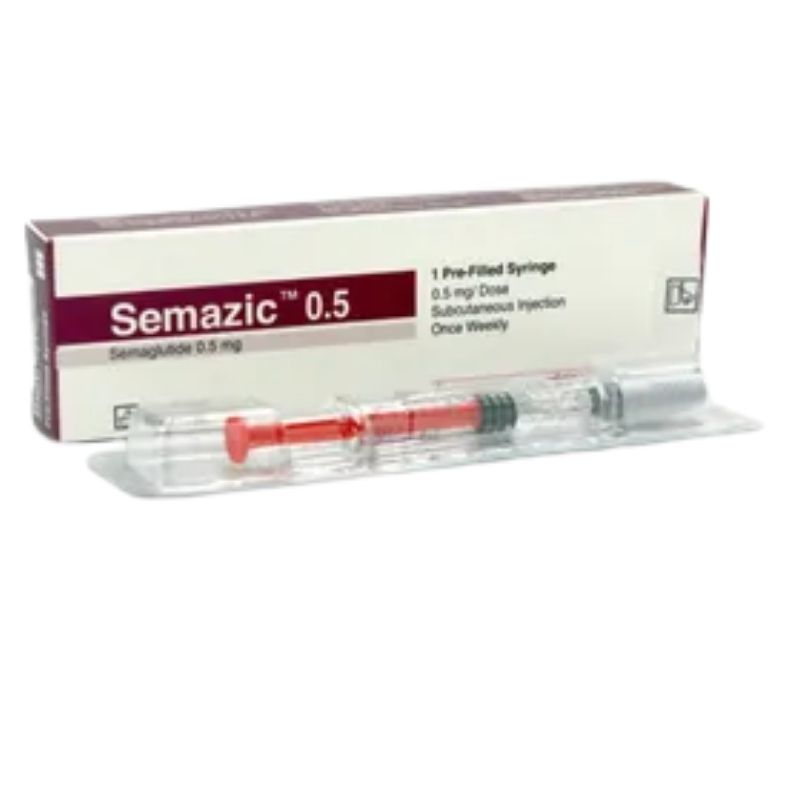
Semazic 0.50 injection – (0.5mg/0.375ml)
600.00৳ Original price was: 600.00৳ .545.00৳ Current price is: 545.00৳ .
Square Pharmaceuticals PLC.
Generic: Semaglutide
1 x 0.50mg pre-filled
| Weight | 0.015 kg |
|---|
Out of stock
0
People watching this product now!
Description
Indication
Type 2 Diabetes Mellitus
Administration
SC Administration Administer SC to abdomen, thigh, or upper arm Administer once weekly, on the same day each week, at any time of the day, with or without meals Day of weekly administration can be changed if necessary as long as the time between 2 doses is at least 2 days (>48 hours) Use a different injection site each week when injecting in the same body region
Adult Dose
Type 2 Diabetes Mellitus Indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus 0.25 mg SC qWeek for 4 weeks initially; THEN increase the dosage to 0.5 mg qWeek If glycemic control not achieved after at least 4 weeks on 0.5-mg dose, can increase to 1 mg qWeek Hepatic impairment No dosage adjustment required
Child Dose
<18 years: Safety and efficacy not established
Renal Dose
Renal impairment No dosage adjustment required
Contraindication
Personal or family history of MTC or in patients with multiple endocrine neoplasia syndrome type 2 (MEN 2) Known hypersensitivity to semaglutide or to any of the product components
Mode of Action
Glucagon-like peptide-1 (GLP-1) agonist Incretins, such as GLP-1, enhance glucose-dependent insulin secretion by pancreatic beta-cells, suppresses inappropriately elevated glucagon secretion, and slows gastric emptying
Precaution
Based on findings in rats and mice, semaglutide may cause thyroid C-cell tumors including MTC, in humans as human relevance of semaglutide-induced rodent thyroid C-cell tumors has not been determined (see Black Box Warnings) In control trials, acute pancreatitis was confirmed by adjudication in 7 semaglutide-treated patients (0.3 cases per 100 patient years); one case of chronic pancreatitis was confirmed in an semaglutide-treated patient; after initiating treatment, monitor for signs and symptoms of pancreatitis (eg, persistent severe abdominal pain, sometimes radiating to the back and which may or may not be accompanied by vomiting) Patients with a history of diabetic…
Side Effect
>10% Nausea (15.8-20.3%) Documented symptomatic hypoglycemia, adjunctive therapy [≤70 mg/dL glucose threshold] (16.7-29.8%) Severe or symptomatic hypoglycemia, adjunctive therapy [≤56 mg/dL glucose threshold] ( 8.3-10.7%) 1-10% Vomiting (5-9.2%) Diarrhea (8.5-8.8%) Abdominal pain (5.7-7.3%) Constipation (3.1-5%) Dyspepsia (2.7-3.5%) Eructation (1.1-2.7%) Documented symptomatic hypoglycemia, monotherapy [≤70 mg/dL glucose threshold] (1.6-3.8%) Flatulence (0.4-1.5%) Gastroesophageal reflux disease (1.5-1.9%) <1% Gastritis (0.4-0.8%) Cholelithiasis (0.4%) Fatigue (>0.4%) Dysgeusia (>0.4%) Dizziness (>0.4%) Injection site reaction (0.2%)
Pregnancy Category Note
Pregnancy Data are limited regarding use in pregnant women Based on animal reproduction studies, there may be potential risks to the fetus from exposure to semaglutide during pregnancy; should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus Discontinue treatment in women at least 2 months before a planned pregnancy, owing to the long washout period for semaglutide Clinical Considerations Poorly controlled diabetes during pregnancy increases maternal risk for diabetic ketoacidosis, preeclampsia, spontaneous abortions, preterm delivery, stillbirth, and delivery complications Poorly controlled diabetes increases fetal risk for major birth defects, stillbirth, and macrosomia-related…
Interaction
Coadministration with insulin secretagogues (eg, sulfonylureas) or insulin may increase the risk of hypoglycemia; consider a lower dose of the secretagogue or insulin to reduce risk of hypoglycemia in this setting Exercise caution when semaglutide is concomitantly administered with oral medications; semaglutide causes a delay of gastric emptying, thereby potentially impacting oral absorption of such medications
Reviews (0)
Only logged in customers who have purchased this product may leave a review.





Reviews
There are no reviews yet.