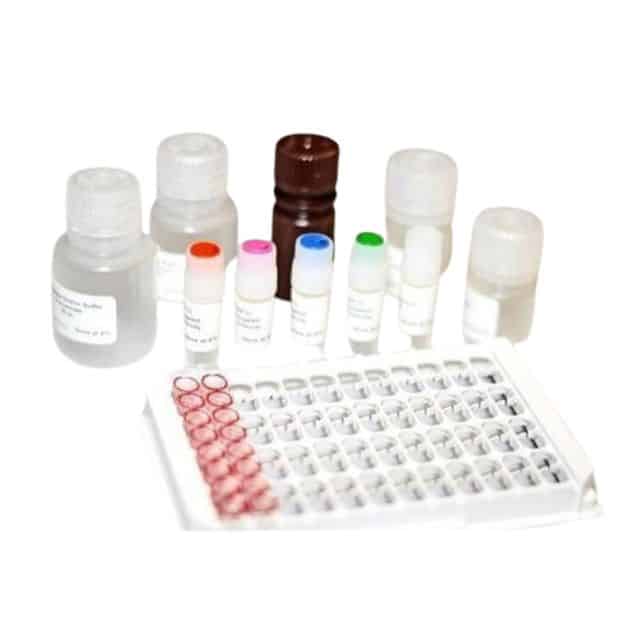
HBsAg Reagent – Jaj international
3,200.00৳ Original price was: 3,200.00৳ .2,800.00৳ Current price is: 2,800.00৳ .
Brand : JAJ
Origin: USA
Packaging Size: Bottle
Number of Test : 96
প্রোডাক্টি কেনার আগে আমাদের সাথে যোগাযোগ করে প্রোডাক্টির বর্তমান দাম সম্পর্কে জেনে নিবে
| Weight | 2 kg |
|---|
HBsAg Reagent – Jaj international
HBsAg is an envelope surface protein of the hepatitis B virus, and a marker of hepatitis B infection. In Japan, an HBsAg reagent was adopted as a blood screening test by the Japan Red Cross in 1972. Furthermore, a highly sensitive HBsAg reagent has been in use since 2008. Blood screening with HBsAg reagent use has contributed to a decrease in hepatitis B infections following blood donation. From the year 2000, the HBsAg reagent requirements have changed according to the progress in hepatitis B treatment. Serum levels of HBsAg and their temporal changes over long periods are used to understand a patient’s clinical state and the effect of nucleoside analogue treatment.
In 2013, we developed a highly sensitive quantitative chemiluminescent enzyme immunoassay (HBsAg-HQ) for HBsAg. Characteristics of the HBsAg-HQ reagent include the use of a pretreatment mixture and anti-HBs antibodies for the inner portion of HBsAg. The detection limit of HBsAg-HQ reagent (zero +3SD) is 0.0006 IU/mL, and the quantitative limit below 10% of the CV is 0.002 IU/mL. Therefore, we configured the measurement range from 0.005 to 150 IU/mL. Diagnostic sensitivity was tested for 10 seroconversion panels with HBsAg-HQ, and HBsAg-HQ yielded the same or higher sensitivity than other immunoassay products.
Only logged in customers who have purchased this product may leave a review.





Reviews
There are no reviews yet.