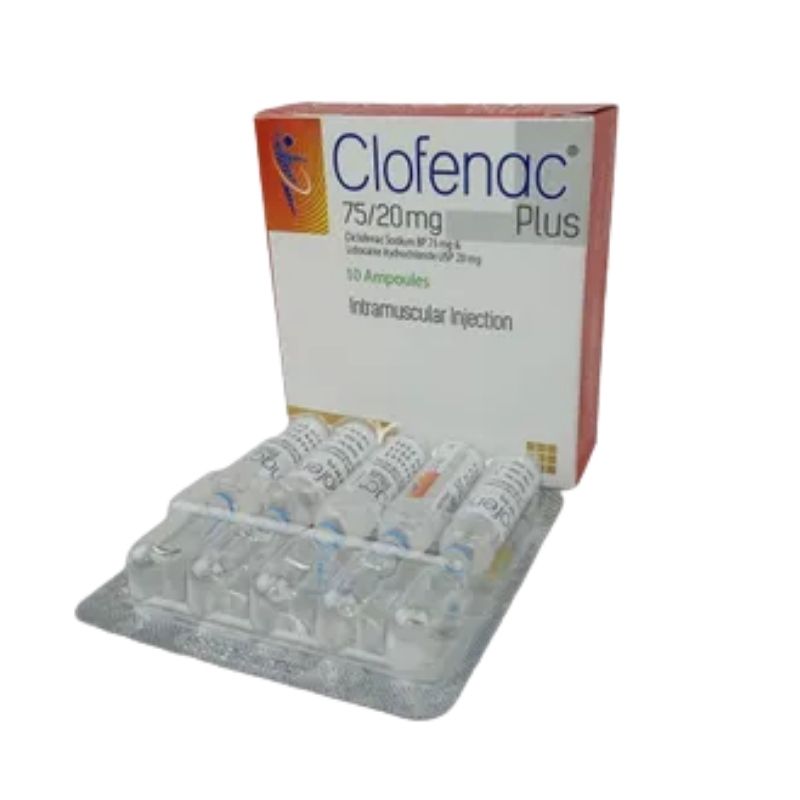
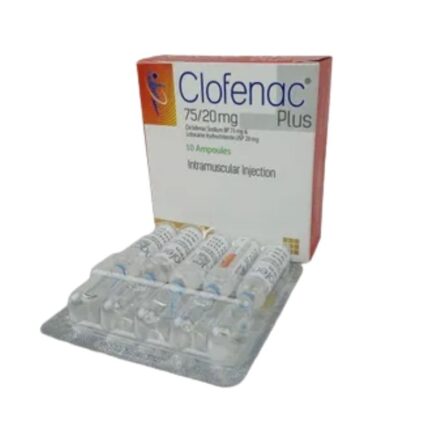
Clofenac Plus Injection – (75mg+20mg/2ml)
16.00৳ Original price was: 16.00৳ .14.00৳ Current price is: 14.00৳ .
Square Pharmaceuticals PLC.
Generic: Diclofenac + Lidocaine Hydrochloride
1 x 2ml Ampoule
| Weight | 0.015 kg |
|---|
0
People watching this product now!
Description
Indication
Rheumatoid arthritis, Osteoarthritis, Ankylosing spondylitis, Pain, Acute gout, Inflammation, Tendinitis, Actinic keratoses, Bursitis
Adult Dose
Intravenous Postoperative pain Adult: As diclofenac Na: 75 mg infusion in glucose 5% or NaCl 0.9% (previously buffered w/ Na bicarbonate) given over 30-120 min or as bolus inj, may repeat after 4-6 hr if necessary. Max period: 2 days. Intramuscular Rheumatoid arthritis; Sprains ; Strains; Tendinitis; Pain and inflammation associated with musculoskeletal and joint disorders ; Bursitis; Acute gout; Dysmenorrhoea Adult: As diclofenac Na: 75 mg once daily, injected into the gluteal muscle, may increase to 75 mg bid in severe conditions. Max period: 2 days. Renal colic Adult: As diclofenac Na: 75 mg, may repeat once after 30…
Contraindication
In patients with active or suspected peptic ulcer or gastrointestinal bleeding, or for those patients in whom attacks of asthma, urticaria or acute rhinitis are precipitated by aspirin or other NSAIDs possessing prostaglandin synthetase inhibitinig activity, it is also contraindicated. Lidocaine is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide-type.
Mode of Action
Diclofenac, a phenylacetic acid derivative is a prototypical NSAID. It has potent anti-inflammatory, analgesic and antipyretic actions. It reversibly inhibits the enzyme, cyclooxygenase, thus resulting in reduced synthesis of prostaglandin precursors. Lidocaine is an amide type local anaesth. It stabilises the neuronal membrane and inhibits Na ion movements, which are necessary for conduction of impulses. In the heart, lidocaine reduces depolarisation of the ventricles during diastole and automaticity in the His-Purkinje system. Duration of action potential and effective refractory period are also reduced.
Precaution
History of gastrointestinal ulceration, haematemesis or melaena, ulcerative colitis, Asthma or history of asthma, Crohn’s disease, bleeding diathesis or haematological abnormalities. Patients with severe hepatic, cardiac or renal insufficiency or the elderly people, Current or previous high blood pressure ( hypertension) should be kept under close surveillance. All patients who are receiving long-term treatment with NSAID agents should be monitored as a precautionary measure (e.g., renal, hepatic function and blood counts).If abnormal liver function tests persist or worsen, clinical signs and symptoms consistent with liver disease develop or if other manifestations occur, Diclofenac sodium should be discontinued. Lactation: Excreted in…
Side Effect
Side-effects of Diclofenac is usually mild and transient. It is generally well tolerated. At the starting of the treatment, however, patients may sometimes complain of gastrointestinal discomfort, epigastria pain, eructation, nausea and Diarrhoea, headache and bleeding sometime may occur. Occasionally skin rash, peripheral oedema and abnormalities of serum transaminase have been reported.Very rarely reported side effects include activation of peptic ulcer, haematemesis or melena, blood dyscrasia (extensive usage). There have been isolated reports of anaphylactoid reactions. The adverse effects due to Lidocaine mainly involve the CNS, are usually of short duration, and are dose related. The CNS reaction may be…
Pregnancy Category Note
Pregnancy category: C; avoid use in late pregnancy (may cause premature closure of ductus arteriosus); category D if >30 weeks after gestation
Interaction
May increase serum levels of methotrexate. Concomitant use w/ other NSAIDs or anticoagulants (e.g. warfarin) is associated w/ higher risk of GI bleeding. Increased risk of nephrotoxicity w/ ciclosporin or triamterene. May increase the risk of developing corneal complications in patients w/ significant pre-existing corneal inflammation when use concomitantly w/ ophth preparation containing corticosteroids. Colestyramine and colestipol reduce the bioavailability of diclofenac. Decreased plasma concentration when administered after sucralfate. Ophth application of diclofenac may reduce the efficacy of ophth acetylcholine and carbachol. May increase serum levels of lithium and digoxin. May increase serum levels w/ cimetidine and propranolol. Increased risk…
Reviews (0)
Only logged in customers who have purchased this product may leave a review.





Reviews
There are no reviews yet.